What Is The Lewis Structure For Br2 - D 2 lone pairs and 1 bonding pair. E none of the above.

How To Draw The Lewis Dot Structure For Br2 Diatomic Bromine Youtube
A 4 lone pairs and 1 bonding pair.

What is the lewis structure for br2. H21 Cl 30 F40 Which molecule below would you expect to have the more polar bond. When metals and non-metals react together they form ionic compoundsThis means that electrons are transferred from one atom to another which creates positively-charged ions cations and negatively-charged. Chemistry questions and answers.
Br2 is nonpolar while HBr is a polar molecule because Br has a higher electronegativity than H. Or if you need more Lewis Dot Structures. The atomic number of Beryllium is.
The electronegativity value for Mg is 12 and the value for O is 35. The Lewis Structure of Beryllium Bromide BeBr2 To begin with the Lewis structure of this compound first we need to know the valence electrons already available in the participating atoms. Neutral Compounds practice you can also practice Lewis Dot Structures.
For the Br 2 Lewis structure there are a total of 14 valence electrons available. Bromine will normally form one covalent bond. Which compound below is a polar molecule AND has a linear molecular geometry.
The Br2Lewis structure is similar to F2 Cl2 and I2since F Cl and I are all in Group 7 and have 7 valence electrons. The Br 2 Lewis structure is similar to F 2 Cl 2 and I 2 since F Cl and I are all in Group 7 and have 7 valence electrons. What is the difficulty of this problem.
The structure uses dots and lines to depict electrons and the bonds between 2 electrons respectively. Bromoether Br2O CID 14513628 - structure chemical names physical and chemical properties classification patents literature biological activities safety. Bromine is a halogen in Group 17 and all of the halogens are non-metals.
Dibromide1- Br2- CID 5460533 - structure chemical names physical and chemical properties classification patents literature biological activities safety. Both of the Br atoms use one of their valence electrons to form the single bonds and the remaining six on each of them form three lone pairs on both. Our tutors rated the difficulty of Draw the Lewis structure for Si Cl 2.
Atoms would have 6 lone electrons on each. Clearly ER-VE 1614 2 electrons. Since the Br atom lies in the 17th group of the periodic table it has seven valence electrons.
How many bonds can Bromine make. Neutral Compounds practice problems. Calcium is a metal in fact it is an alkaline earth metal in Group 2 of the periodic table.
State whether HBr is polar or nonpolar. C 2 lone pairs and 3 bonding pairs. Look in the periodic table.
- Answers Well Be has 2 valence electrons and Br has 7 so 272 16 Now the structure would be B----Be----B. State whether Br2 is polar or nonpolar. A neutral bromine atom would also have 35 electrons.
Consider the following electronegativity values. Divide by 2 this gives SP1 Continue Reading Lyndon Tabuco Answered 3 years ago 41K views View upvotes Sponsored by Alexa Stories. Whats the correct Lewis structure for Br2.
What is Lewis structure of bromine. The atomic number for bromine is 35 which means it has 35 protons in its atomic nuclei. In the BrO3- Lewis structure Bromine Br is the least electronegative and goes in.
15 The Lewis structure for carbon monoxide is CO This structures shows. Start studying Chemistry - Chapter 10. Br2 is the molecular formula name for bromine gas dibromine or molecular bromine.
Bromine has the Lewis structure of an single. For the Br2Lewis structure there are a total of 14 valence electrons available. What is the structure of Br2.
Lewis Structure of Br2 A compounds Lewis Structure depicts the arrangement of its underlying valence shell electrons. What is the molecular geometry shape of the molecule for BBrs Q2. How do you draw a lewis structure for XeBr2Cl2 what is the formal charge of Xe what is the molecular geometry.
ER 28 16 Then calculate how many electrons you actually have. How many covalent bonds can bromine form. A step-by-step explanation of how to draw the MgBr2 Lewis Dot StructureFor MgBr2 we have an ionic compound and we need to take that into account when we dra.
Br 2 is sometimes called diatomic Bromine since it is made up of two Bromine atoms. In Lewis theory everything is paired so divide by two giving TP 142 7 total electron pairs. Learn vocabulary terms and more with flashcards games and other study tools.
You need to reconcile the discrepancy between ER and VE by sharing electrons. First calculate the number of electrons required ER to give everybody an octet. What Is The Lewis Structure Of Br2.
What shape is Br2. Draw Lewis structure for BBr Q2. The bromine molecule is a diatomic molecule with a single Br-Br bond.
What is the Lewis dot structure for br2. Based on these values what type of bond is expected for a. What is the Lewis structure for BeBr2.
How many electrons does Br2. Br2is sometimes called diatomic Bromine since it is made up of two Bromine atoms. B 4 lone pairs and 3 bonding pairs.

Draw Lewis Structures For The Following Molecules And Ions A Br2 B H3o C N2 D Hf Study Com

Br2 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
How To Draw The Lewis Dot Structure For Br The Element Bromine Youtube

How To Draw The Lewis Dot Structure For Br2 Diatomic Bromine Youtube
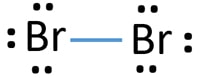
Bromine Br2 Lewis Structure

Video Lewis Dot Structure For Br2

Write Out The Electron Configuration For Neutral Bromine Draw The Lewis Dot Structure Which Depiction Allows For Better Understanding Of Electrons Involved In Bonding Explain Your Reasoning Socratic

Br2 Lewis Structure Bromine Gas Youtube
What Is The Lewis Dot Structure For Br2 Quora
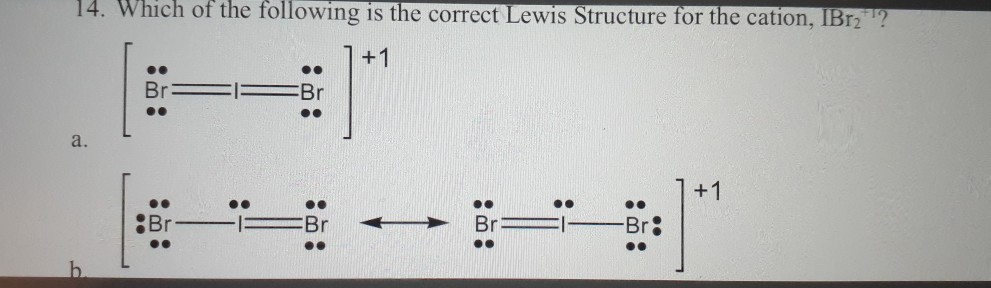
Solved 14 Which Of The Following Is The Correct Lewis Chegg Com
Bromine Home

Solution Draw The Lewis Structure For Si Clutch Prep

Chapter 8 Covalent Bonding Ppt Download
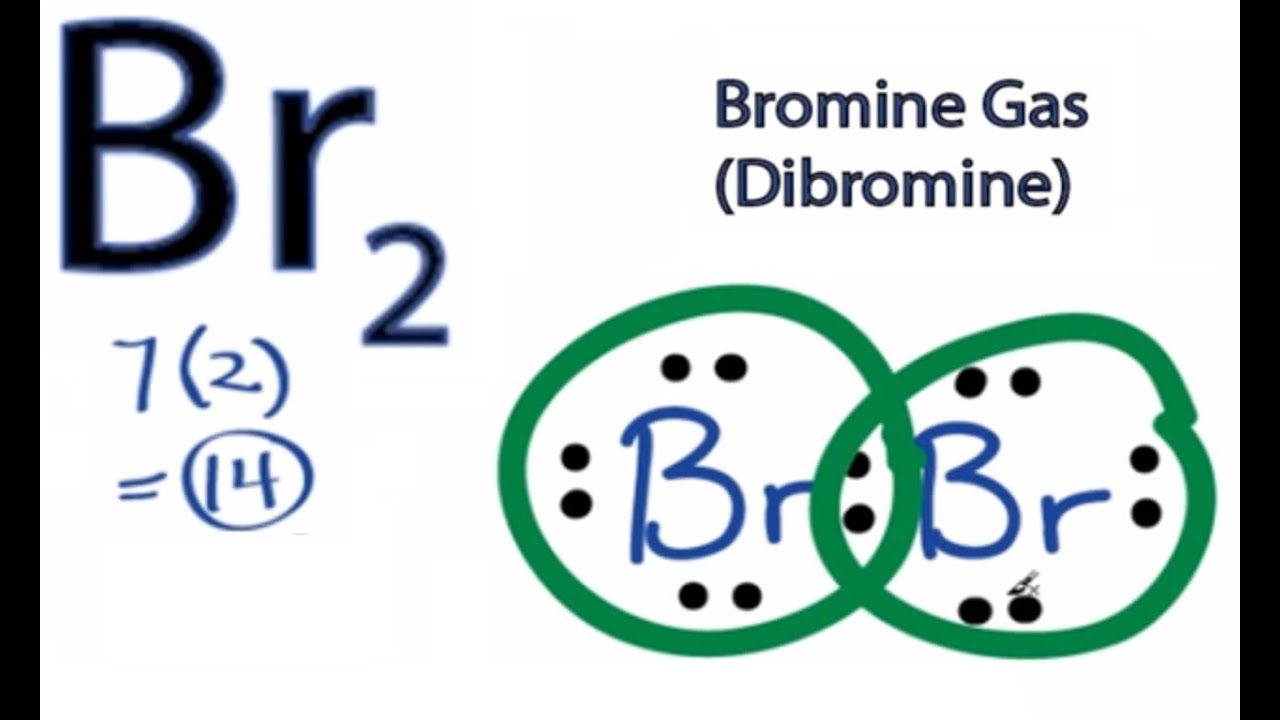
Br2 Lewis Structure How To Draw The Lewis Dot Structure For Dibromine Youtube